商品圖片僅供參考,選購時請再留意商品規格
商品圖片僅供參考,選購時請再留意商品規格

商品圖片僅供參考,選購時請再留意商品規格
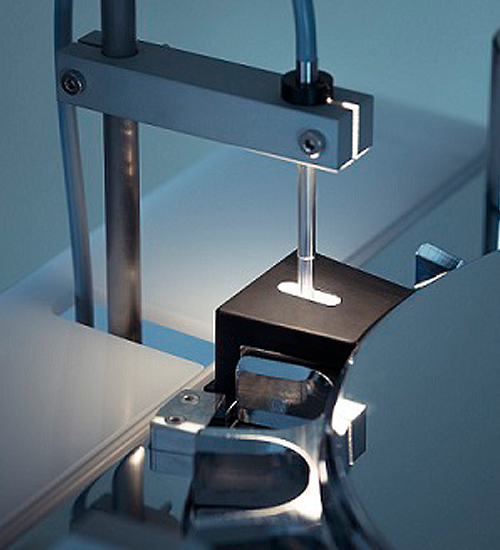
Charles Ischi AG IPC.line(+VisioNIR) UTS Extended
商品包含多種型號及規格,請點選「立即選購規格」挑選
月結報帳 / 信用卡 / 銀行轉帳
宅配快速到貨,滿 NT$ 999 元即享免運!
商品介紹
產品簡介
Nowadays, batches easily reach sizes of >1,000,000 tablets. However, only 30 tablets are used for content uniformity (CU) release testing. But there can be many more...
The UTS-Extended with integrated VisioNIR LS technology can check for these physical-chemical characteristics. In some cases, the thickness or the bizarre shape of the tablets does not allow the transmission measurement.
The solution is the possibility to switch via a multiplexer from transmission to reflection mode to enable the user to measure each available tablet on the market. For detailed homogeneity inspection requirements, the tablet can be area-scanned in reflection mode. The resulting plot is an image of the tablet showing the active pharmaceutical ingredient (API) content distribution in the tablet. On the other hand, the mean content of this multi-spot measurement can be used for an overall statement of the API content of the tablet.
From a quality point of view, the RAS (rapid area scan) measurement provides much more solid results. The reliable trend monitoring visualizes the CU prediction value of each sampled tablet. Outliers can be safely identified. The UTS-Extended is dedicated to real time release (RTR).
The UTS-Extended with integrated VisioNIR LS technology can check for these physical-chemical characteristics. In some cases, the thickness or the bizarre shape of the tablets does not allow the transmission measurement.
The solution is the possibility to switch via a multiplexer from transmission to reflection mode to enable the user to measure each available tablet on the market. For detailed homogeneity inspection requirements, the tablet can be area-scanned in reflection mode. The resulting plot is an image of the tablet showing the active pharmaceutical ingredient (API) content distribution in the tablet. On the other hand, the mean content of this multi-spot measurement can be used for an overall statement of the API content of the tablet.
From a quality point of view, the RAS (rapid area scan) measurement provides much more solid results. The reliable trend monitoring visualizes the CU prediction value of each sampled tablet. Outliers can be safely identified. The UTS-Extended is dedicated to real time release (RTR).
產品特色
以上圖片及相關資訊由 Charles Ischi AG提供。
若有現場安裝、說明或特殊配送需求,歡迎事前與科研市集聯繫。部分商品因供應條件限制,可能無法提供相關服務,敬請見諒。